Abstract
Introduction
Immunoglobulin light-chain amyloidosis (AL amyloidosis) results from abnormal production and deposition of misfolded immunoglobulin light chains (LC). Currently, the treatment approach is plasma cell-directed therapies (PCD) that inhibit abnormal LC production. Here we present a systematic review of new anti-amyloid antibodies (AA-Ab) like CAEL-101, NEOD001, and Anti-serum amyloid P component (SAP) that target misfolded proteins and enhance their removal.
Material and Methods:
A comprehensive literature search was done on the following databases; PubMed, Embase, Cochrane, and Clinical Trials.gov. Articles involving CAEL-101, NEOD001, and Anti-SAP in patients (pts) with AL amyloidosis were included. Conference abstracts including ASH and ASCO annual meetings until 2022 were searched. Data extraction was done on a separate excel file.
Results:
CAEL-101 Monoclonal Antibody:
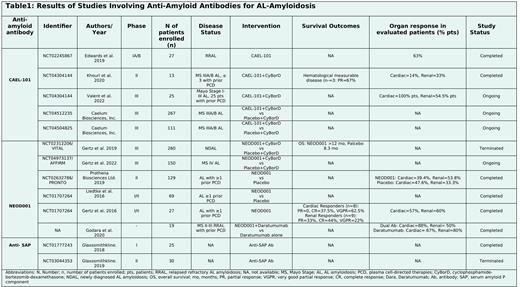
27 pts with relapsed refractory AL amyloidosis (RRAL) received CAEL-101 (0.5-500mg/m2). 63% pts who manifested cardiac (CD), renal (RD), hepatic or gastrointestinal disease showed organ response (OR). No drug-related (DR) adverse event (AEs) was reported (Edwards 2019). 13 pts with Mayo Stage (MS) IIIA/B AL amyloidosis received CAEL-101+standard of care (SoC) cyclophosphamide-bortezomib-dexamethasone (CyBorD). 6 pts received the maximum tolerated dose (MTD) of 1000mg/m2. Partial response (PR) was achieved by 2/3 pts. No DRAEs were reported (Khouri 2020). In a dose-escalation study, CAEL-101 was administered in 25 pts with MS I-III AL amyloidosis with SoC. OR was seen in 30% pts with CD and 100% pts with RD. Treatment-emergent (TE) AEs (nausea, constipation, diarrhea, or fatigue) and DRAEs were experienced by 24 and 6 pts respectively. A phase 3 trial is ongoing (Valent 2022). Two randomized phase 3 studies (NCT04512235, NCT04504825) with 267 and 111 pts respectively with MS IIIA/B AL amyloidosis are currently ongoing.
NEOD001 (Birtamimab) Monoclonal Antibody:
260 pts with newly diagnosed, AL amyloidosis were evaluated in VITAL study involving treatment arm (Rx) A NEOD001+SoC and Rx B placebo+SoC. Median overall survival (OS) in MS IV was >12 months (mo) and 8.3 mo respectively. No statistically significant difference was found. Study was terminated when futility analysis revealed a hazard ratio (HR) of 0.835 (95% CI 0.5799-1.2011, p=0.330) favoring NEOD001. Post-hoc analyses suggested a potential survival benefit of NEOD001 for MS IV AL amyloidosis (HR 0.498). Similar TEAEs (fatigue, nausea, peripheral edema, constipation, and diarrhea) were observed in both arms (Gertz 2019). A phase 3 AFFIRM trial (Gertz 2022) is currently ongoing that enrolled 150 pts with MS IV AL amyloidosis who will receive either NEOD001+SoC or placebo+SoC. Another trial, PRONTO was discontinued in 2018 as it could not reach its endpoints. Liedtke,2016 evaluated the response of NEOD001 in 69 pts with AL amyloidosis with atleast 1 PCD treatment. OR was seen in 53% pts with CD and 63% pts with RD. NEOD001 resulted in high OR which was not dependent on time since previous chemotherapy or PCD. 27 pts with AL amyloidosis were evaluated by Gertz 2016. MTD for NEOD001 was 24mg/kg. 4 pts had grade (G) ³3 AEs including hyponatremia, diverticulitis, and pneumonia. OR was observed in 57% pts with CD and 60% pts with RD. To evaluate the efficacy of dual Ab especially with CD in pts with AL amyloidosis, Godara 2020 enrolled 19 pts with MS II-III RRAL who had received prior PCD. Rx A and B involved 9 and 10 pts who received Daratumumab(Dara)+NEOD001 vs Dara alone respectively. The CD and RD in Rx A vs B were as follows: 89% and 44% vs 70% and 80% respectively. Hematologic response observed in both arms was 100% (33 days) vs 80% (75 days) respectively. OR (cardiac and renal) observed in both arms was 88% and 50% vs 67% and 80% respectively. 6 G-2 AEs were reported with dual Ab (infusion reaction, skin rash, cough). 2 pts with Dara experienced respiratory symptoms.
Anti-SAP Antibody:
Trials for Anti-SAP Ab are either completed with non-satisfactory results or terminated owing to the change in risk/benefit profile by certain data (NCT01777243, NCT03044353).
Conclusion:
CAEL-101 and NEOD001 are novel therapies for advanced AL amyloidosis with mild to moderate TEAEs. The ongoing trials need a longer follow-up to further elucidate the safety and efficacy of AA-Ab (Table1).
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal